Business
Our strengths
With nearly 100 years of history,
Our passion drives Japan’s cosmetic ODM industry.
As a Japanese cosmetic ODM manufacturer with nearly a century of history, we have continuously moved forward. Here, we would like to introduce our strengths and our ongoing commitment to the growth of the cosmetics industry.

With a Century of Trust,
Innovating the Future of Cosmetics
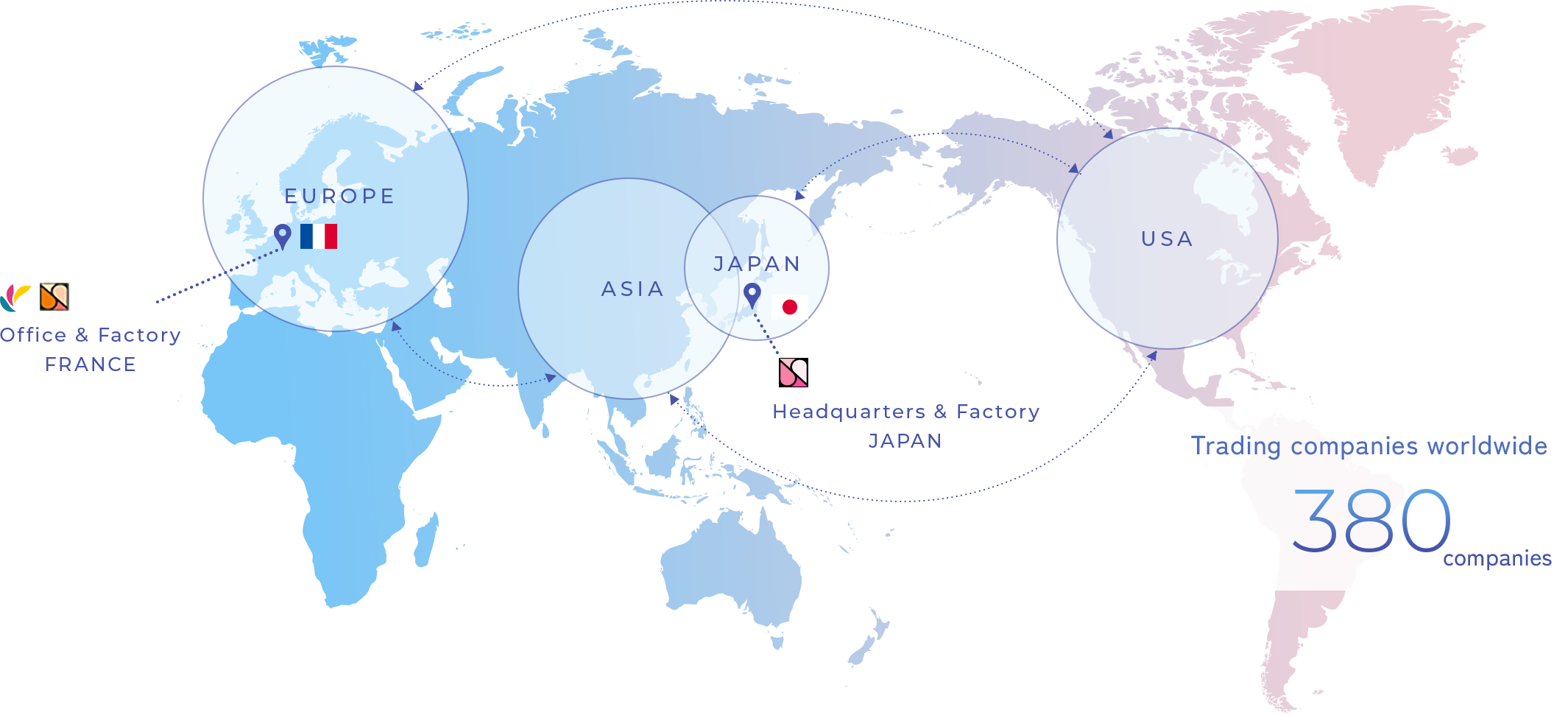
At Nippon Shikizai, we handle numerous products for renowned brands in Europe and the United States.
We acquired THEPENIER PHARMA & COSMETICS in 2000 and Nippon Shikizai France in 2017,
enabling us to leverage the strengths of both ‘Made in Japan’ and ‘Made in France’ for the development
and production of cosmetics. Through collaborative formulation development, technology
and personnel exchanges, and business activities among the three group companies,
we are expanding our business and advancing our globalization.
ODM of Nippon Shikizai Nippon Shikizai is equipped with a comprehensive system that supports all stages from product design to the final product.

Nippon Shikizai is an ODM manufacturer specialized in product development.
Although we do not have our own brand and do not appear on the market, the products we manufacture are transformed into our customers’ brands and are loved by people in Japan and around the world.
Our products and packaging, which integrate the latest technologies and trends, have been highly appreciated by many customers engaged in cosmetic research and development, production technology, and product planning for many years due to their high quality.
We are able to maintain the highly qualified production thanks to the production system that provides total support, as shown on the left.
Research and Development
Challenges in R&D for a Sustainable Future:
Our Commitment to ‘Clean Beauty First

In our group’s research and development, we strive to promptly provide products that meet the diversified and advanced needs and requirements of the broad markets in the cosmetic fields, quasi-drugs, and U.S. OTC. Our main policy is to actively propose new formulas based on both basic and applied research.
To achieve this, we prioritize technical development that is friendly to people and the environment. We continue to commit to ‘clean beauty’ with a strong focus on sustainability.
Even a small lipstick contains numerous innovations in ingredients, packaging, bulk, processes, and reliable safety information.
In each cosmetic product, we pursue clean beauty by integrating “functionality that beautifully enhances customers around the world” with “technological developments that maximize kindness to the Earth”.
Research and Development Structure
To develop innovative and high-quality products, various organizations collaborate to maximize the utilization of technology and knowledge. We deliver safe and reliable products that incorporate cutting-edge technologies.

Development Policy
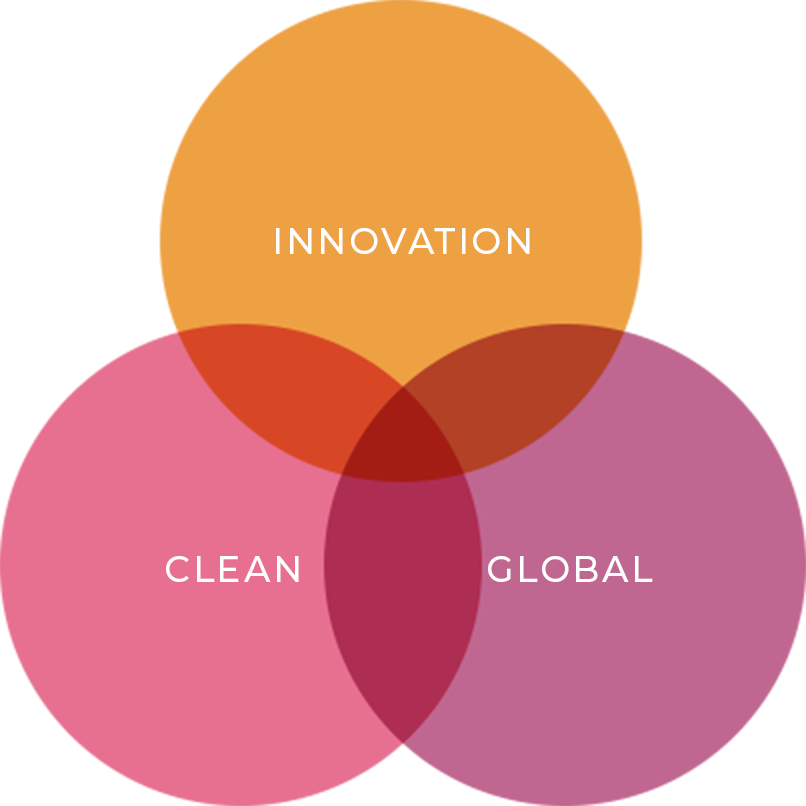
By integrating the three core concepts—INNOVATION, GLOBAL, and CLEAN—
we create a synergistic effect that enables us to develop products
tailored to our customers’ diverse and intricate needs
Based on the insights and experience gained throughout our long history since the company founded, we develop products with a unique approach, actively incorporating new technologies, ingredients, and daily research outcomes, without being constrained by traditional frameworks. We not only respond to ever-changing market needs but also propose products that anticipate future trends. With our expertise rooted in the history of Nippon Shikizai and innovative product development free from common assumptions, we provide one and only value that gives an impression and emotion.
We have been working with not only Japanese clients but also various overseas clients. Our product development complies with the cosmetic regulations of each country, ensuring that we deliver products suitable for each market. Furthermore, many global trends in cosmetics originate from the EU. As we have a subsidiary in France, we can directly gather market trends and regulatory information, consistently reflecting the latest information in our product development.
CLEAN BEAUTY is a concept that focuses on the transparency, safety, and environmental friendliness of cosmetic ingredients and manufacturing processes. Developing sustainable products comes with various challenges. We have promptly responded to this trend, promoting environmentally friendly product development while ensuring quality and safety. We aim to pursue the beauty that cosmetics can create, while minimizing environmental impact and building a sustainable future.
Research Publication- Beyond Product Development
We also actively participate in presenting our research findings. The International Federation of Societies of Cosmetic Chemists (IFSCC) is a global organization for individuals who study cosmetic science. At the IFSCC Congress, cosmetic scientists from around the world gather to present new research findings and technologies, thereby deepening their knowledge collectively. It is a valuable opportunity to present their research on an international stage, and our young researchers have presented their research findings and received high praise.
Technical Development
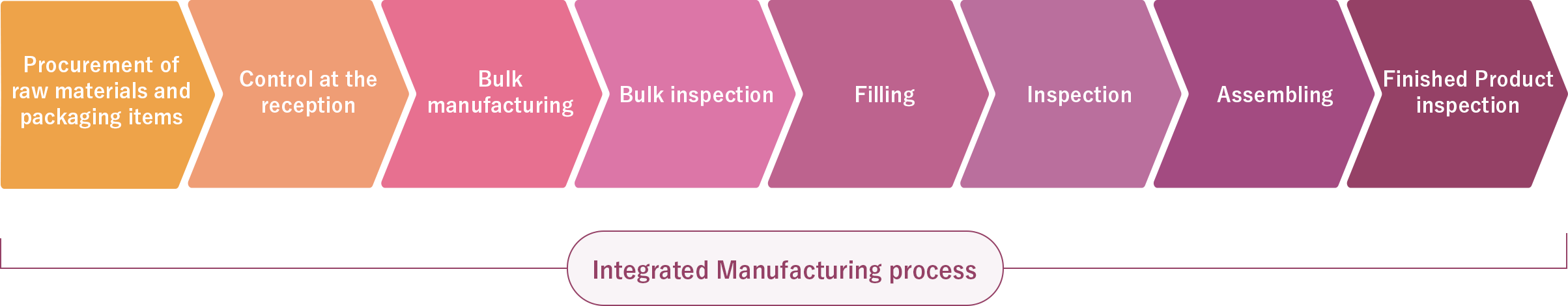
We are capable of accommodating various shipment formats to meet our customers’ demands. Not only offering integrated manufacturing processes, we also offer split production processes such as manufacturing followed by filling, or bulk production only.

To bring our customers’ ideas and innovations to life, we use our own specially developed production equipment to achieve the mass production of high-quality products that require advanced technology. Our precise and flexible production technology allows us to meet a wide range of needs, from large-scale production to small-scale, multi-variety production. Additionally, we provide technical support to our facility in France while maintaining a system to promptly gather the latest information from France and other overseas sources.
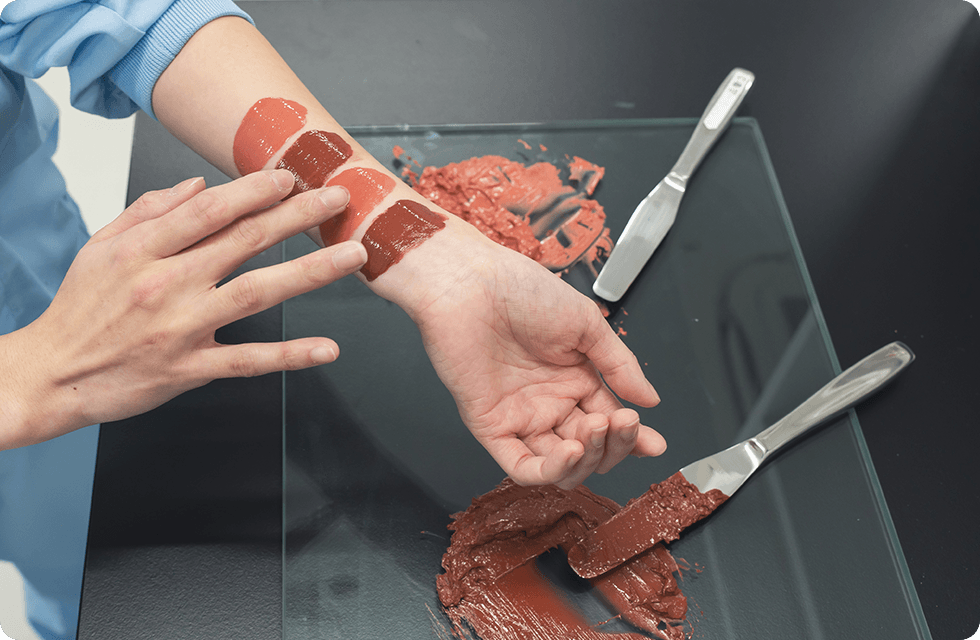
Cosmetic production requires the technical expertise of skilled professionals. Especially the shade matching in the bulk manufacturing, high technical skills are essential. In some cases, there are 50 different shades with subtle differences between each shade, we carry out the shade matching by taking into account the various conditions such as the particle size of pigment and the characteristics of each raw material as well as the temperature during the mixing process.

From in-house developed products to customized products that meet customer requests. Based on nearly 100 years of experience in cosmetic production technology, we bring these to life at our Zama and Tsukuba factories in Japan.
Every step of the process—from bulk production, container selection, filling methods, to optimal packaging and wrapping—incorporates a perspective on technological development. Additionally, daily improvements are made to achieve mass production, continuously striving to enhance quality and productivity.
Quality Assurance
As Your Best Partner
We Commit to Ensuring Satisfactory Quality Assurance

At Nippon Shikizai, we carry out thorough quality inspections from the adoption of new raw materials to formulation development and production activities. This ensures that we have established a robust quality assurance system that provides complete confidence for all our customers.
Quality Assurance System
Our Tsukuba factory is registered with the United States Food and Drug Administration (FDA) as a manufacturing site for OTC (over-the-counter) drugs and undergoes regular audits. This allows us to manufacture products like sunscreens, which are considered OTC drugs in U.S. markets.

Our factories are equipped to analyze various active ingredients that are approved for their effectiveness and effects in Japan. We will propose active ingredients that match the desired effectiveness and effects of our clients, and assist with everything from formulation design to obtaining manufacturing and sales approval.

Companies that meet international standards for quality and safety in the manufacturing processes of cosmetics are certified. Given that it is legislated within the EU and compliance is required worldwide, both our Zama and Tsukuba factories have obtained this certification.

Quality Control System

Since regulations are becoming increasingly stricter concerning heavy metals in cosmetics both domestically and internationally, we at Nippon Shikizai have invested in ICP-MS (Inductively Coupled Plasma Mass Spectrometer) to further strengthen our impurity control system from raw materials to finished products.

Only personnel who have passed Nippon Shikizai’s strict certification system are allowed to perform sensory tests for ‘color tone,’ ‘smell,’ and ‘feel’.
- Physical properties (viscosity, hardness, specific gravity, breakage test, drop point, pH)
- Analysis (HPLC, GC, FT-IR, Karl Fischer moisture titration, residue on ignition)
- Microbiological testing